
Scientifically flawed
Animal toxicity tests are crude, subjectively assessed and the results can vary depending upon the species, age, sex and condition of individual animals. One international study that examined the results of rat and mouse LD50 (Lethal Dose 50%) tests for 50 chemicals found that these tests were able to predict toxicity in humans with only 65% accuracy. (1) (see page 17) Dr Robert Sharpe, research chemist, states, "The LD50 for digitoxin in rats is 670 times that in cats, whilst for the antifungal substance antimycin, the LD50 in chickens is 30-80 times greater than in pigeons and mallards. The LD50 of thiourea in the wild Norway rat is 450 times greater than in the Hopkin's stain of rat." (2) Manufacturers are simply asked to conduct whatever tests are appropriate, in their opinion, to establish that their cosmetics or household products are safe. Even the environmental conditions in a laboratory can affect results. The LD50 results for the same chemical can vary widely between different laboratories. It is hardly surprising then to learn that results from animal tests are often difficult to apply to humans. Many substances tested safely on animals have proven to be dangerous to humans and vice versa.
The real reasons
Animal tests were crudely developed as long ago as the 1920s and became commonplace in the 1940s. Scientists are familiar and comfortable with the animal-based techniques they have been using for years. It is always difficult to change the status quo. Companies continue to test on animals for legal protection. Animal testing is designed to protect a manufacturer against legal claims by consumers. The irony is that the defence "we have safety-tested our products on animals" only becomes relevant when that testing fails to detect a potentially dangerous substance and a consumer is injured. There is no actual legal requirement for animal testing. Manufacturers are simply asked to conduct whatever tests are appropriate, in their opinion, to establish that their cosmetics or household products are safe. The use of animals in laboratories is supported by a very large and powerful industry. It includes contract testing laboratories, the suppliers of cages, equipment, animals, and infrastructure. Alternatives to animal testing Today, many cosmetic and household product companies have turned their backs on animal testing and begun taking advantage of the many sophisticated non-animal test methods available, which range from cell and tissue cultures to computerised "structure-activity relationship" models. Human cell culture tests have been found to predict toxicity in humans with much greater accuracy than animal tests. (1) R. Roggeband et al., "Eye Irritation Responses in Rabbit and Man After Single Applications of Equal Volumes of Undiluted Model Liquid Detergent Products," Food and Chemical Toxicology, 38 (2000): 727-734. (2) Dr Robert Sharpe, "The Cruel Deception".
Toxicity tests
Chemical toxicity (poisoning) testing on animals involves subjecting animals to different levels of potentially toxic substances via different routes of exposure in order to assess how and in which way they are affected.Many products are tested to see if they will cause damage to the skin or eyes. This approach to chemical testing, which uses animals and is mainly observational, subjective and descriptive, is extremely crude. Animal tests tell us little about why a substance is toxic, as the results tend to demonstrate effects rather than causes of toxicity. The test results are difficult to extrapolate from laboratory conditions to real life exposure of humans. Their credibility is based on established use rather than proven predictive value. Most standard animal tests were developed decades ago and have either never been validated, or have actually failed retrospective validation (for example, the Draize eye test, the Lethal Dose 50% test and carcinogenicity).
Types of tests
Repeated dose & sub-chronic toxicity To assess the toxic effects on the whole body of repeated sub-lethal doses of a chemical (i.e. the dosing is intended to show poisoning effects on internal organs, the nervous system etc. up to but not including death). Forty to 80 rats are usually used per chemical and/or 32 dogs can be used as a second 'non-rodent' species. The animals are repeatedly dosed with a chemical over a period of 28-90 days. This is usually done orally (force-feeding with a syringe or tube) but may also be administered dermally or inhaled. At the end of their ordeal, the animals are killed and their tissues examined pathologically and biochemically. Symptoms can include blood pressure changes, excessive salivation, anaemia, aggression, muscle weakness, hair loss, internal organ damage, pilo-erection (hair standing on end), vomiting (in dogs), tremors, diarrhea, coma and occasionally death. Chronic toxicity To assess the effects of long-term chemical exposure for significant periods of the animal's life span. As with repeated dose and sub-chronic toxicity, one rodent species (usually 160 rats) and one non-rodent species (usually 32 dogs but can involve primates) will be subjected to exposure via force feeding, dosing in food, through the skin or via forced inhalation. The length of the study is at least 12 months and as much as two years. Symptoms can include blood pressure changes, loss of appetite, aggression, restlessness, muscle weakness, excessive salivation, internal organ damage, pilo-erection, vomiting (dogs), tremors, bloody diarrhea, coma and death. Mutagenicity To identify any mutagenic effects (genetic mutations) of the chemical either on the rapidly dividing cells of the bone marrow or on the nuclei of blood cells. Usually using 40 rats, mice or hamsters, the test material is administered either orally or by injection into the body cavity. Developmental toxicity (teratogenicity) To assess whether the test substance, when ingested, causes malformations in the embryo. This usually involves at least 80 pregnant rats or 48 pregnant rabbits subjected to a graduated dose or concentration of the test substance during the period of organ formation in the developing embryo. Three dose levels are given where the highest is sufficient to evoke minor changes in the mother (for example loss of weight). Dosing is usually oral and the embryos are killed and examined for gross or more subtle anatomical changes. The mothers endure daily force-feeding by stomach tube throughout pregnancy and may experience poor weight gain, loss of appetite, nasal discharge, pilo-erection, hair loss, diarrhea, dehydration and occasionally death. The unborn animals can also be damaged by the chemical. Reproductive toxicity (mammals) To identify any effect of a chemical upon the male or female reproductive capacity. Approximately 100 female rats (80 pregnant) and 40 male rats will be used. They are given graduated doses (usually orally) during their reproductive cycles. Assessment is made of post-administration effects on fertility, pregnancy and maternal effects (feeding and nesting behaviour). These animals endure daily force-feeding by stomach tube and can also experience poor weight gain, loss of appetite, nasal discharge, pilo-erection, hair loss, diarrhea, dehydration and death. Carcinogenicity Used to detect any cancerous changes as a result of exposure to a substance. It uses at least 400 very young rats or mice per substance and involves dosing the animals as soon after weaning as possible and thereafter for the rest of their lives. The animals are usually force-fed the substance but chemicals can also be painted on the skin or delivered by forced inhalation. The symptoms of this slow chemical poisoning include cancerous tumours, lethargy, nausea and death; autopsy will also reveal any tissue or internal organ damage. Toxicokinetics Used to follow the time course of toxic (poisoning) effects and to find out how quickly or easily a substance is absorbed from the gut or through the skin into the bloodstream, how long it is in circulation and how it is metabolised and excreted. Doses are either single or multiple. At least eight healthy young animals are used (can be rodents and dogs) per chemical and dosed orally, via the skin or forced inhalation. For the duration of the test the animals are isolated in small, barren, metal metabolism cages so that their urine and faeces can be collected separately. Some animals also have tubes implanted into their bile ducts. Symptoms include loss of appetite, lethargy, nasal discharge, pilo-erection (hair standing on end), hair loss, diarrhea, dehydration and vomiting (in dogs). At the end of the experiment all the animals are killed and examined for the accumulation of test substances in target organs. Eco-toxicity Example, acute toxicity in fish. Eco-toxicity tests are conducted to measure a chemical's effects on the environment and wildlife. In fish toxicity, the test chemical is put in the water of large fish-tanks and records are kept of how many fish die as a result of slow poisoning over a number of days. LD test LD stands for Lethal Dose - the dose of a substance that will kill a percentage of the test animals. A single dose of the test substance is usually placed directly into the stomachs of animals via a tube. Different groups of animals are given increasing doses of the test substance to see which dose will kill them. Symptoms of toxic substances include abdominal pain, cramps, convulsions, vomiting (in some species), diarrhea, paralysis, breathing difficulties and bleeding ulcers. Rats and mice are used, but sometimes dogs and rabbits are also included.
Problems with the LD test
Clearly this is a very cruel test. Gerhard Zbinden, one of the world's best known toxicologists, has called it "a ritual mass execution of animals". It gives no information on treating human poisoning. It is an unreliable way of predicting risk to humans because the results are altered by so many factors. Firstly, there are huge species differences. Ten-fold species differences are common. For example, the LD50 for paracetamol was 250-400mg/kg in mice and hamsters. Death was caused by liver damage. However, in rats the LD was 1000mg/kg, and there was no sign of liver damage.
Skin & eye irritancy tests
The application of test substances onto the skin or into the eye of an animal.
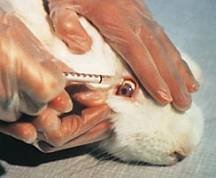
Draize eye irritancy test
The traditional method for testing irritation and damage to the eye is the Draize test. The test substance is placed in the eyes of conscious rabbits, who are either held in stocks or have plastic collars around their neck so that they can't rub their eyes with their paws. Researchers look for signs of redness, swelling, discharge and ulceration to determine how irritating the substance is. The rabbits are killed at the end of the test. The outer layer of the eye, the cornea, is one of the most sensitive tissues in the body. It is richly supplied with nerve endings, which is why any irritation or damage is extremely painful. Everyone knows how uncomfortable it is to get something like shampoo or onion in the eye. We quickly wash it out. In comparison, the suffering of rabbits is greater, firstly because some of the substances tested are more irritating, and secondly because the rabbits can't wash their eyes.
Skin irritancy test
To test whether a substance irritates the skin, sections of the animals' backs are shaved and sometimes abraded. The substance is applied to the skin and covered with gauze patches. Researchers then look for signs of redness, inflammation, weeping or scabs to determine how irritating the substance is. An irritating product can make the skin red raw, which is obviously very painful. Rabbits are usually used for this test. At the end of the test they are killed.
Problems with irritancy tests
The first problem with these tests is that they are very, very cruel. Irritation to the skin and especially the eye can be excruciatingly painful. However, the tests are also inaccurate. In one study the same 12 substances were tested for eye irritancy in 24 well-established laboratories. Since the same substances were being tested, using the same method, and the same species of animal, you would expect scores for the degree of eye injury to be similar. On the contrary, scores varied between rabbits in the same laboratory and varied widely between laboratories. Some substances that were rated as most irritating by some laboratories were rated as least irritating by others. There are a number of differences between rabbit and human eyes:
- rabbits have a third eyelid
- rabbits produce less tear fluid to wash away irritants
- rabbits have a more alkaline eye (human pH 7.1-7.3, rabbit pH 8.2)
- rabbits have a thinner cornea (human 0.51mm, rabbit 0.37mm).
One study compared the results of animal tests using rabbits and monkeys with information regarding accidental human exposure to products. All animal tests, especially the standard Draize test, overestimated how irritating a product was to the human eye. An experienced toxicologist has concluded: "No single animal species has been found to model exactly for the human eye, either in anatomical terms or in response to irritation". There are also considerable differences between human and rabbit skin. When 12 substances were tested on human and rabbit skin, results were similar only for the two most irritating products. The remaining ten products were irritating to the rabbits but not the humans. In another study, a range of household products and industrial chemicals were tested on the skin of rabbits, guinea pigs and humans. Only four of the products were non-irritating in all three species. However, 12 products were more irritating in one or both of the animal species than in humans. A further three products were less irritating in one or both animal species than in humans. The researchers concluded: "Neither the rabbit nor the guinea pig provides an accurate model for human skin. The skin responses of these animals differ in both degree and in kind from those of human skin."
In the case of cosmetics, the manufacturer could choose ingredients that have been used for a long time, and so are likely to be safe. If the chemical is a new one, the first test could be the QSAR computer analysis to predict its likely irritancy. In the next stage, a number of in vitro (test tube) tests could be used. Some tests are better than others for substances of a certain form, for example liquid rather than solid, and for substances of a certain chemical class, for example alcohols rather than oils. The aim would be to choose the best set of tests for a particular substance. Doing more than one test is not a problem because in vitro tests are so much faster and cheaper than animal tests. For example, an Agarose Diffusion Test takes 24 hours per product, whereas a Draize test takes at least 3 days per product and costs 10 times more to carry out. If the product is shown to be safe by this set of tests, it can then be trialed by human volunteers. This is the final and best test of all. By going through this series of steps products can be guaranteed to be safe without the suffering that is currently inflicted on animals.
Alternative irritancy tests
Many different in vitro (test tube) systems have been suggested as alternatives to animal irritancy tests. The following are only a few examples.
Eytex(TM)
This test for eye irritancy uses a vegetable valign="top" protein extracted from jack beans. Like the cornea of the eye, this clear protein gel becomes cloudy when in contact with an irritating substance. In the Draize test, people have to estimate the degree of damage caused, that is, how swollen or red part of the rabbit's eye is. This system isn't very accurate. In the Eytex test, the degree of cloudiness ("damage") can be measured by a machine, a spectrophotometer, which is much more reliable.
Reconstructed human epidermis
This is a multi-layered human skin grown in the laboratory, which can be used to test skin irritancy. It is sold commercially under trade names such as Skin Squared(TM) and Episkin(TM). There are various ways of measuring damage when an irritating product is applied to this test skin. For example, cells can be examined under the microscope, membrane damage can be assessed by leakage of enzymes, or inflammation can be determined by release of interleukins. Whatever method is used, the result can be measured accurately, unlike in animal studies where observers estimate the degree of swelling or redness.
Corneal cell lines
The SIRC is a continuous cell line of rabbit corneal cells. These are cells that are now grown in the laboratory, and no further rabbits are killed. When 6 shampoos were tested on these cells, there was very good agreement with Draize results. The test assessed how much of a substance was needed to kill half the cells. Obviously, the less of a substance that is needed to produce this result, the more damaging it is. However, to avoid species differences it would obviously be preferable to use human cells. One problem with using cells from human corneas has been that these cells don't live for very long. Now researchers have found a way of not only increasing the number of these cells, but also extending their life span so that they can be studied in more detail. Researchers used human corneas from an eye bank to grow the cells. This cell culture can be used not only to study eye irritation, but also wound healing, parasite infection, and radiation damage in the eye.
Neutral Red Uptake Test
Normal cells in culture readily absorb and hold this neutral red dye. When the cell membrane, or the lysosomes inside the cell are damaged by an irritating chemical, dye will be lost through the leaky membranes. Less dye will remain in the cell. A spectrophotometer is used to accurately measure how much has been lost.
Agarose Diffusion Test
The problem with cell cultures such as those in the Neutral Red Uptake Test is that the cells are in fluid, so only soluble substances can be tested. In the Agarose Diffusion Test a small amount of agarose (a seaweed extract) is added to form a gel layer. Some of the test substance is placed on a small piece of filter paper, which is then placed on the agarose. The substance diffuses through the agarose into the cell culture below. The irritancy of the substance is assessed by measuring the area, in millimetres, of dead cells under the filter paper, that is, cells that have lost their neutral red dye.
Microphysiometer
An irritating product will produce changes in the functioning of cells. The microphysiometer is an instrument that detects very small changes in cell metabolism by measuring changes in the pH of the cell culture nutrient fluid (changes in lactate, CO2 production).
Computer modelling
Expert computer systems can be used to predict the irritancy of new substances on the basis of what is already known about the irritancy of substances with a similar chemical structure. This approach is known as Quantitative Structure-Activity Relationship, or QSAR for short. The molecular structure of known substances is entered into a computer database. Particular chemical structures are linked to particular kinds of chemical activity, in this case irritancy. When a new substance is entered, the expert system tries to match its molecular structure to others in the database. If it finds a close similarity, it predicts that the new substance has the same level of irritancy.
Human studies
Some cosmetics companies already use human volunteers to test new formulations. This is the most reliable test of all. Human irritancy can be assessed through patch testing, where test substances are placed on small areas of the upper back and covered with a patch for 2 days.
Alternative toxicity
In 1989 the Scandinavian Society for Cell Toxicology organised a large international study of alternative methods. This study was called Multicenter Evaluation of In Vitro Cytotoxicity (MEIC), and involved laboratories in many different countries. A list of 50 chemicals was selected for testing by the Swedish Poison Information Centre. Chemicals for which there was good human data were chosen. In other words, for these chemicals it was known how much it would take to kill a human from the results of accidents or suicides. When the MEIC project closed in 1996, 59 laboratories from all over the world had submitted results. There were 29 laboratories that had tested all 50 chemicals. In total, 61 different in vitro (test tube) methods were used. The researchers compared the lethal doses (LD) taken by humans with the LD50 in animals. They also compared the lethal concentration (LC) in the bloodstream of people who died with the concentration that produced a 50% reduction in the growth of cell cultures (IC50). Results from the final evaluation showed that human cell culture tests were more accurate than animal LD50 tests. As MEIC director, Dr Bjorn Ekwall, has commented, cell culture systems can still be further improved, but animal tests can't. By the year 2003 a new project, called EDIT, aims to develop and publish a set of about 6 in vitro tests that will predict human toxicity with 90% accuracy. EDIT also aims to develop a set of tests to predict long-term toxicity. Tests are being worked on where repeated doses of a chemical are added to the cell culture for 6 weeks. Human cell cultures have several advantages in predicting toxicity:
- They are human and so avoid species differences;
- They can be taken from the tissue that a particular test chemical is most likely to affect, for example the skin, or the liver;
- They allow researchers to study how a substance causes damage to the cells, that is, why it is toxic;
- They avoid causing pain and death to animals.
- Human tissues for testing are becoming more available in the USA and Europe, although less so in Australia.
- There are companies that market cell lines from normal tissues and from tumours. For example:
- The American Type Culture Collection has available over 2300 animal and human cell lines.
- Companies such as Clonetics market cell cultures derived from human skin, cardiovascular system, brain, respiratory system, kidneys and muscles (visit the Clonetics web site).
- Researchers can collect their own human tissue, for example, blood from volunteers or skin from plastic surgery. For many tissues, though, it is easier to use tissue banks.
- In the USA, the National Disease Research Interchange in Philadelphia collects tissues removed during operations and from donors, and distributes them to researchers (visit the NDRI web site and look at About Us and Our Mission and History).
- In the UK, the University of Leicester is doing the same. They use organs that are not suitable valign="top" for transplantation and would otherwise be destroyed.



tl;dr, but cool story sis.
ReplyDeleteI would never want to live in a world without animal testing. Let's just fucking kill 3,000 people each time we want our next major medical breakthrough. And the cosmetics and medicine would cost exponentially more.
Nice copy-pasting though.
Animal testing for cosmetics is disgusting.
ReplyDeleteI can understand the medical point of view; if it saves lives, then I'm not against it.
But testing on animals for beauty products and other unnecessary things is just cruel - if you wouldn't do it to a human, why do it to an animal?
I buy all my detergents and hygenic things from here: http://etgronnerehjem.no it's a bit more pricey than what you'd find at the store, but well worth it.
ReplyDeleteFor cosmetic alternatives, like razors and stuff, you can check http://e-beautyshop.no or http://veganessentials.com ^__^