Scientifically flawed
Animal toxicity tests are crude, subjectively assessed and the results can vary depending upon the species, age, sex and condition of individual animals. One international study that examined the results of rat and mouse LD50 (Lethal Dose 50%) tests for 50 chemicals found that these tests were able to predict toxicity in humans with only 65% accuracy. (1) (see page 17) Dr Robert Sharpe, research chemist, states, "The LD50 for digitoxin in rats is 670 times that in cats, whilst for the antifungal substance antimycin, the LD50 in chickens is 30-80 times greater than in pigeons and mallards. The LD50 of thiourea in the wild Norway rat is 450 times greater than in the Hopkin's stain of rat." (2) Manufacturers are simply asked to conduct whatever tests are appropriate, in their opinion, to establish that their cosmetics or household products are safe. Even the environmental conditions in a laboratory can affect results. The LD50 results for the same chemical can vary widely between different laboratories. It is hardly surprising then to learn that results from animal tests are often difficult to apply to humans. Many substances tested safely on animals have proven to be dangerous to humans and vice versa.
The real reasons
Animal tests were crudely developed as long ago as the 1920s and became commonplace in the 1940s. Scientists are familiar and comfortable with the animal-based techniques they have been using for years. It is always difficult to change the status quo. Companies continue to test on animals for legal protection. Animal testing is designed to protect a manufacturer against legal claims by consumers. The irony is that the defence "we have safety-tested our products on animals" only becomes relevant when that testing fails to detect a potentially dangerous substance and a consumer is injured. There is no actual legal requirement for animal testing. Manufacturers are simply asked to conduct whatever tests are appropriate, in their opinion, to establish that their cosmetics or household products are safe. The use of animals in laboratories is supported by a very large and powerful industry. It includes contract testing laboratories, the suppliers of cages, equipment, animals, and infrastructure. Alternatives to animal testing Today, many cosmetic and household product companies have turned their backs on animal testing and begun taking advantage of the many sophisticated non-animal test methods available, which range from cell and tissue cultures to computerised "structure-activity relationship" models. Human cell culture tests have been found to predict toxicity in humans with much greater accuracy than animal tests. (1) R. Roggeband et al., "Eye Irritation Responses in Rabbit and Man After Single Applications of Equal Volumes of Undiluted Model Liquid Detergent Products," Food and Chemical Toxicology, 38 (2000): 727-734. (2) Dr Robert Sharpe, "The Cruel Deception".
Toxicity tests
Chemical toxicity (poisoning) testing on animals involves subjecting animals to different levels of potentially toxic substances via different routes of exposure in order to assess how and in which way they are affected.Many products are tested to see if they will cause damage to the skin or eyes. This approach to chemical testing, which uses animals and is mainly observational, subjective and descriptive, is extremely crude. Animal tests tell us little about why a substance is toxic, as the results tend to demonstrate effects rather than causes of toxicity. The test results are difficult to extrapolate from laboratory conditions to real life exposure of humans. Their credibility is based on established use rather than proven predictive value. Most standard animal tests were developed decades ago and have either never been validated, or have actually failed retrospective validation (for example, the Draize eye test, the Lethal Dose 50% test and carcinogenicity).
Types of tests
Repeated dose & sub-chronic toxicity To assess the toxic effects on the whole body of repeated sub-lethal doses of a chemical (i.e. the dosing is intended to show poisoning effects on internal organs, the nervous system etc. up to but not including death). Forty to 80 rats are usually used per chemical and/or 32 dogs can be used as a second 'non-rodent' species. The animals are repeatedly dosed with a chemical over a period of 28-90 days. This is usually done orally (force-feeding with a syringe or tube) but may also be administered dermally or inhaled. At the end of their ordeal, the animals are killed and their tissues examined pathologically and biochemically. Symptoms can include blood pressure changes, excessive salivation, anaemia, aggression, muscle weakness, hair loss, internal organ damage, pilo-erection (hair standing on end), vomiting (in dogs), tremors, diarrhea, coma and occasionally death. Chronic toxicity To assess the effects of long-term chemical exposure for significant periods of the animal's life span. As with repeated dose and sub-chronic toxicity, one rodent species (usually 160 rats) and one non-rodent species (usually 32 dogs but can involve primates) will be subjected to exposure via force feeding, dosing in food, through the skin or via forced inhalation. The length of the study is at least 12 months and as much as two years. Symptoms can include blood pressure changes, loss of appetite, aggression, restlessness, muscle weakness, excessive salivation, internal organ damage, pilo-erection, vomiting (dogs), tremors, bloody diarrhea, coma and death. Mutagenicity To identify any mutagenic effects (genetic mutations) of the chemical either on the rapidly dividing cells of the bone marrow or on the nuclei of blood cells. Usually using 40 rats, mice or hamsters, the test material is administered either orally or by injection into the body cavity. Developmental toxicity (teratogenicity) To assess whether the test substance, when ingested, causes malformations in the embryo. This usually involves at least 80 pregnant rats or 48 pregnant rabbits subjected to a graduated dose or concentration of the test substance during the period of organ formation in the developing embryo. Three dose levels are given where the highest is sufficient to evoke minor changes in the mother (for example loss of weight). Dosing is usually oral and the embryos are killed and examined for gross or more subtle anatomical changes. The mothers endure daily force-feeding by stomach tube throughout pregnancy and may experience poor weight gain, loss of appetite, nasal discharge, pilo-erection, hair loss, diarrhea, dehydration and occasionally death. The unborn animals can also be damaged by the chemical. Reproductive toxicity (mammals) To identify any effect of a chemical upon the male or female reproductive capacity. Approximately 100 female rats (80 pregnant) and 40 male rats will be used. They are given graduated doses (usually orally) during their reproductive cycles. Assessment is made of post-administration effects on fertility, pregnancy and maternal effects (feeding and nesting behaviour). These animals endure daily force-feeding by stomach tube and can also experience poor weight gain, loss of appetite, nasal discharge, pilo-erection, hair loss, diarrhea, dehydration and death. Carcinogenicity Used to detect any cancerous changes as a result of exposure to a substance. It uses at least 400 very young rats or mice per substance and involves dosing the animals as soon after weaning as possible and thereafter for the rest of their lives. The animals are usually force-fed the substance but chemicals can also be painted on the skin or delivered by forced inhalation. The symptoms of this slow chemical poisoning include cancerous tumours, lethargy, nausea and death; autopsy will also reveal any tissue or internal organ damage. Toxicokinetics Used to follow the time course of toxic (poisoning) effects and to find out how quickly or easily a substance is absorbed from the gut or through the skin into the bloodstream, how long it is in circulation and how it is metabolised and excreted. Doses are either single or multiple. At least eight healthy young animals are used (can be rodents and dogs) per chemical and dosed orally, via the skin or forced inhalation. For the duration of the test the animals are isolated in small, barren, metal metabolism cages so that their urine and faeces can be collected separately. Some animals also have tubes implanted into their bile ducts. Symptoms include loss of appetite, lethargy, nasal discharge, pilo-erection (hair standing on end), hair loss, diarrhea, dehydration and vomiting (in dogs). At the end of the experiment all the animals are killed and examined for the accumulation of test substances in target organs. Eco-toxicity Example, acute toxicity in fish. Eco-toxicity tests are conducted to measure a chemical's effects on the environment and wildlife. In fish toxicity, the test chemical is put in the water of large fish-tanks and records are kept of how many fish die as a result of slow poisoning over a number of days. LD test LD stands for Lethal Dose - the dose of a substance that will kill a percentage of the test animals. A single dose of the test substance is usually placed directly into the stomachs of animals via a tube. Different groups of animals are given increasing doses of the test substance to see which dose will kill them. Symptoms of toxic substances include abdominal pain, cramps, convulsions, vomiting (in some species), diarrhea, paralysis, breathing difficulties and bleeding ulcers. Rats and mice are used, but sometimes dogs and rabbits are also included.
Problems with the LD test
Clearly this is a very cruel test. Gerhard Zbinden, one of the world's best known toxicologists, has called it "a ritual mass execution of animals". It gives no information on treating human poisoning. It is an unreliable way of predicting risk to humans because the results are altered by so many factors. Firstly, there are huge species differences. Ten-fold species differences are common. For example, the LD50 for paracetamol was 250-400mg/kg in mice and hamsters. Death was caused by liver damage. However, in rats the LD was 1000mg/kg, and there was no sign of liver damage.
Skin & eye irritancy tests
The application of test substances onto the skin or into the eye of an animal.
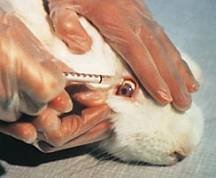
Draize eye irritancy test
The traditional method for testing irritation and damage to the eye is the Draize test. The test substance is placed in the eyes of conscious rabbits, who are either held in stocks or have plastic collars around their neck so that they can't rub their eyes with their paws. Researchers look for signs of redness, swelling, discharge and ulceration to determine how irritating the substance is. The rabbits are killed at the end of the test. The outer layer of the eye, the cornea, is one of the most sensitive tissues in the body. It is richly supplied with nerve endings, which is why any irritation or damage is extremely painful. Everyone knows how uncomfortable it is to get something like shampoo or onion in the eye. We quickly wash it out. In comparison, the suffering of rabbits is greater, firstly because some of the substances tested are more irritating, and secondly because the rabbits can't wash their eyes.
Skin irritancy test
To test whether a substance irritates the skin, sections of the animals' backs are shaved and sometimes abraded. The substance is applied to the skin and covered with gauze patches. Researchers then look for signs of redness, inflammation, weeping or scabs to determine how irritating the substance is. An irritating product can make the skin red raw, which is obviously very painful. Rabbits are usually used for this test. At the end of the test they are killed.
Problems with irritancy tests
The first problem with these tests is that they are very, very cruel. Irritation to the skin and especially the eye can be excruciatingly painful. However, the tests are also inaccurate. In one study the same 12 substances were tested for eye irritancy in 24 well-established laboratories. Since the same substances were being tested, using the same method, and the same species of animal, you would expect scores for the degree of eye injury to be similar. On the contrary, scores varied between rabbits in the same laboratory and varied widely between laboratories. Some substances that were rated as most irritating by some laboratories were rated as least irritating by others. There are a number of differences between rabbit and human eyes:
- rabbits have a third eyelid
- rabbits produce less tear fluid to wash away irritants
- rabbits have a more alkaline eye (human pH 7.1-7.3, rabbit pH 8.2)
- rabbits have a thinner cornea (human 0.51mm, rabbit 0.37mm).
One study compared the results of animal tests using rabbits and monkeys with information regarding accidental human exposure to products. All animal tests, especially the standard Draize test, overestimated how irritating a product was to the human eye. An experienced toxicologist has concluded: "No single animal species has been found to model exactly for the human eye, either in anatomical terms or in response to irritation". There are also considerable differences between human and rabbit skin. When 12 substances were tested on human and rabbit skin, results were similar only for the two most irritating products. The remaining ten products were irritating to the rabbits but not the humans. In another study, a range of household products and industrial chemicals were tested on the skin of rabbits, guinea pigs and humans. Only four of the products were non-irritating in all three species. However, 12 products were more irritating in one or both of the animal species than in humans. A further three products were less irritating in one or both animal species than in humans. The researchers concluded: "Neither the rabbit nor the guinea pig provides an accurate model for human skin. The skin responses of these animals differ in both degree and in kind from those of human skin."
( http://www.choosecrueltyfree.org.au/ )